Abstract
The bay scallop, Argopecten irradians, is a species of major commercial, cultural, and ecological importance. It is endemic to the eastern coast of the United States, but has also been introduced to China, where it supports a significant aquaculture industry. Here, we provide an annotated chromosome-level reference genome assembly for the bay scallop, assembled using PacBio and Hi-C data. The total genome size is 845.9 Mb, distributed over 1,503 scaffolds with a scaffold N50 of 44.3 Mb. The majority (92.9%) of the assembled genome is contained within the 16 largest scaffolds, corresponding to the 16 chromosomes confirmed by Hi-C analysis. The assembly also includes the complete mitochondrial genome. Approximately 36.2% of the genome consists of repetitive elements. The BUSCO analysis showed a completeness of 96.2%. We identified 33,772 protein-coding genes. This genome assembly will be a valuable resource for future research on evolutionary dynamics, adaptive mechanisms, and will support genome-assisted breeding, contributing to the conservation and management of this iconic species in the face of environmental and pathogenic challenges.
Similar content being viewed by others
Background & Summary
Bivalves (Bivalvia), a class of mollusks, encompass a vast array of species that are integral to marine and freshwater ecosystems1,2. Most members of the class are filter feeders that help mitigate eutrophication and improve water quality, and many bivalve species serve as bioindicators of environmental health and represent a vital food source for humans and other animals3,4,5. In 2020, over 16 million tons of bivalves were produced from farming activities worldwide6, representing a commercial value of nearly 30 billion US$. Among the bivalves, the family Pectinidae, commonly known as scallops, is of particular interest due to their ecological significance and economic value7. The Pectinidae family comprises over 300 species distributed worldwide, with members known for their distinctive fan-shaped shells8. This family includes the bay scallop, Argopecten irradians, a species that has attracted considerable attention due to its unique biological traits and commercial importance. The species displays remarkable polymorphism in shell color patterns (Fig. 1), relatively short lifespan, and exhibits unique locomotion through rapid shell clapping9,10,11.
The bay scallop naturally inhabits shallow coastal waters along the eastern coast of North America, from New England to the Gulf of Mexico12,13. They prefer estuaries and bays with relatively high salinity, water depths of 0.3 to 0.6 m at low tide, and seagrass beds14. Small batches of A. irradians were introduced from the United States to China in the 1980s and 1990s and served to establish a very successful aquaculture production, yielding about 1 million tons annually15,16,17.
The genomic study of bivalves, particularly within the Pectinidae family, has lagged behind other groups such as oysters18 and mussels19, leaving a substantial gap in our understanding of their genetic diversity and adaptive potential. Scallop genomic assemblies have been previously generated for Mizuhopecten yessoensis20, Chlamys farreri21, and Argopecten purpuratus22, but chromosome-scale scallop genome assemblies available in open databases are currently limited to Pecten maximus23 and Mimachlamys varia24. Recently, the draft genomes of bay scallop subspecies (irradians and concentricus) cultivated in China have been sequenced25. However, these genomes are scaffolded and reflect a complex introduction history due to their aquaculture origin. Prior studies also reported reduced allele diversity in bay scallop populations in China, suggesting that the limited man-made stock introductions may have yielded a bottleneck in genetic diversity among continuously cultured stocks26. This complexity, in addition to the draft nature of the current assemblies, may pose challenges for using these genomes in genomic and environmental research related to the species’ natural habitat.
The availability of a high-quality genome assembly for A. irradians marks a significant advancement in bivalve genomics, promising to shed light on the complex biological processes that underpin their survival and productivity. Especially important is the fact that since 2019, the bay scallop population in New York has suffered from catastrophic and recurring summer mortality events that has devastated the commercial fishery. This mortality is associated with annual outbreaks of an undescribed apicomplexan parasite, recently dubbed Bay Scallop Marosporida (BSM)27,28. This study presents the first chromosome-level genome assembly of A. irradians, achieved using PacBio sequencing and Hi-C technology. The assembled genome measures 845.9 Mb, featuring a scaffold N50 length of 44.3 Mb. A total of 33,772 protein-coding genes were predicted within the A. irradians genome. This high-quality assembly, derived from specimens in their native habitat in New York, provides a crucial genomic resource for advancing genetic improvement and elucidating the functional genes and molecular mechanisms underlying the peculiar traits of the bay scallop. In contrast to the existing A. irradians draft genome assemblies, this newly assembled genome offers significant improvements in both resolution and completeness, resulting in a more contiguous and comprehensive assembly. The genome was generated from a scallop produced by a breeding program that uses wild broodstock to maintain a broader genetic base, thereby reducing the bottleneck effect commonly observed in aquaculture stocks.
Methods
Sample collection and genome sequencing
The reference genome was generated from an adult scallop (62 mm) collected from a first-generation aquacultured stock bred by Cornell Cooperative Extension from wild broodstock harvested from Orient Harbor, New York, USA (41.137904, −72.315392). The scallop was transported to the laboratory on ice for processing, where the testis was dissected and immediately used for DNA extraction using standard phenol-chloroform-isoamyl alcohol (PCI) extraction29. In parallel, the adductor muscle was dissected and flash-frozen in liquid nitrogen before transfer to a −80 °C freezer for subsequent Hi-C sequencing. High-molecular-weight gDNA obtained from testis and subsequently purified was prepared for PacBio single-molecule real-time (SMRT) sequencing using the Express Template Preparation Kit 2.0 (Pacific Biosciences) according to the manufacturer’s protocol. Approximately 2 μg of gDNA was sheared to create 10-kb libraries using Covaris g-TUBEs, followed by concentration using 0.45X AMPure PB beads (Pacific Biosciences). This sheared gDNA was enzymatically treated to remove single stranded overhangs and to repair nicked DNA templates. An end repair and A-tailing reaction further prepared the sample by repairing blunt ends and polyadenylating each template. SMRTbell adapters were then ligated to each template and 0.45X AMPure PB beads were used for purification to remove small fragments and excess reagents. Size selection of the purified SMRTbell libraries was performed at 6–50 kb using the BluePippin system on 0.75% agarose cassettes and S1 ladders according to the manufacturer’s specifications (Sage Science (Beverly, Massachusetts, USA)). The final size-selected library was annealed to sequencing primer v4 and coupled to sequencing polymerase 1.0, then sequenced on two 8 M SMRT cells on the Sequel II system, each with a 20-hour movie. This resulted in a total of 9,919,395 reads with an average length of 14,207 bp. The flash-frozen adductor muscle was processed for Hi-C library construction using an Arima Genomics Hi-C Kit (San Diego, California, USA) according to the manufacturer’s guidelines. This Hi-C library was then sequenced on a single lane of an Illumina HiSeqX PE150, resulting in a total of 779,291,520 paired-end reads.
Transcriptome sequencing
Transcriptomic data were generated from kidney samples derived from a total of 137 wild and aquacultured scallops collected from Orient Harbor, New York, and used in laboratory experiments or deployed in Flanders Bay (40.917634, −72.593486). RNA was extracted using the NucleoSpin® RNA Plus RNA isolation kit (Macherey-Nagel, Düren, Germany). RNA quantity and quality were checked spectrophotometrically (NanoDrop® ND-1000, Thermo Fisher Scientific, Wilmington, Delaware, USA). Library preparation and sequencing were performed by Novogene Corporation (UC Davis, Sacramento, California, USA). Sample quality control measures implemented by Novogene rely mainly on RNA Nano 6000 Assay Kit using the Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, California, USA). RNA-seq libraries were prepared using 1 µg RNA using NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, Massachusetts USA). Library fragments were purified with AMPure XP system (Beckman Coulter, Beverly, California, USA). Then 3 µl USER Enzyme (New England Biolabs, USA) was used with size-selected, adaptor-ligated cDNA at 37 °C for 15 min followed by 5 min at 95 °C before PCR. Then PCR was performed with Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. Finally, PCR products were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using PE Cluster Kit cBot-HS (Illumina) before sequencing on an Illumina platform where 150 bp paired-end reads were generated.
Genome assembly
The initial assembly was generated from sequences derived from all PacBio reads after adaptor removal using BBmap’s removesmartbell.sh script. Strategies recommended by Guiglielmoni et al.30 were adopted, using the Raven assembler31 with default parameters to produce a 1Gb-size assembly. Potential uncollapsed haplotypes were removed using Purge Haplotigs32. A polishing process was then conducted using HyPo33. Scaffolding of this assembly was further achieved using Hi-C data. Hi-C reads were processed with hicstuff34 with the parameters --enzyme DpnII,HinfI --iterative. This processing pipeline incorporated a mapping step against the contigs using Bowtie 235. instaGRAAL36 was run with --level 5 --cycles 100 --coverage-std 1 --neighborhood 5 parameters, with further automatic curation from instagraal-polish script. Blobtools37 was run with default parameters on the final scallop assembly to detect potential contamination. For this, Illumina reads were mapped on the assembly using the BWA mem algorithm38 and BLASTn v. 2.11.039 was run against the NT database from NCBI40, providing input to Blobtools. This workflow generated a chromosome-level genome assembly of the bay scallop that contains a total of 845.9 Mb distributed over 1,503 scaffolds with a GC content of 35.6%. The scaffolds have an N50 of 44.3 Mb (L50 = 8 scaffolds) and an N90 of 34.5 Mb (L90 = 16 scaffolds) (Table 1). Confirmatory Hi-C analysis revealed the presence of 16 chromosome pairs in A. irradians (Fig. 2).
Chromosomal organization and synteny in Argopecten irradians NY. (a) A circular genomic map illustrating 16 chromosomes with color-coded segments (I), detailing GC content (II), gene (III) and repeated sequence density (IV). Interconnecting lines (V) represent syntenic blocks and conserved genomic regions across the chromosomes. (b) Contact map of the A. irradians genome assembly. Map generated from Hi-C data showing sequence interaction points in chromosomes (red dots). The color bar indicates contact density. (c) Genomic collinearity between A. irradians and the king scallop, P. maximus.
The majority (92.9%) of our assembled genome is contained within the 16 largest scaffolds which ranges from 75.5 Mb to 34.5 Mb. In addition to the nuclear genome, the complete mitochondrial genome of 16,414 bp was successfully assembled.
Genome annotation
RepeatModeler v. 2.0.441 was used to identify repetitive elements in the genome of A. irradians. Tandem repeats were identified using Tandem Repeats Finder v. 4.0.1042 with recommended parameters. Repeats and low-complexity DNA sequences were masked using RepeatMasker v. 4.1.543. The repeat content of the A. irradians genome (Table 2) is similar to those reported in Pecten maximus23 and Mizuhopecten yessoensis20. Total interspersed repeats represent 36.2% of the A. irradians genome, which is closer to the 38.9% observed in M. yessoensis and higher than the 25.8% seen in P. maximus.
Prediction of protein-coding genes was based on ab initio gene predictions, homology-based predictions, and transcriptome-based predictions. Ab initio predictions were performed by Augustus v. 3.544, GlimmerHMM v. 3.0.245, and SNAP46. For homology-based prediction, GeMoMa v. 1.947 was used to annotate the gene models in A. irradians NY using amino acid sequences from P. maximus23, M. yessoensis20, A. irradians (subspecies irradians and concentricus)25 genomes and TOGA48 was used with the human genome (hg38) as the reference. For RNA-seq-based prediction, the clean RNA-seq reads were aligned to the assembled genome using HISAT2 v2.2.149 and were assembled by StringTie v. 2.2.050 with the default parameters, and then TransDecoder v5.5.0 (https://github.com/TransDecoder/TransDecoder) and PASA v. 2.4.151 were jointly used for final coding-gene prediction. All gene structures predicted by the above methods were integrated into a nonredundant gene set using EVidenceModeler v. 1.1.151. The weight value was set to 10 for high-quality RNA-seq transcripts, 5 for high-quality homologous proteins, and 1 for ab initio predicted transcripts. Finally, the resulting protein models were finally functionally annotated by integrating the annotation information from InterProScan v. 5.63–95.052, KOALA (KEGG Orthology And Links Annotation)53, and the eggNOG-mapper v. 2.0.154,55. Noncoding RNA was annotated using RNAmmer v. 1.256 for rRNA, tRNAscan-SE v. 2.057 for tRNA and the cmscan module in Infernal v. 1.1.258 for miRNA, snRNA and snoRNA. A comprehensive annotation of protein-coding sequences was achieved through a multifaceted approach that integrated de novo gene prediction, protein homology searches, and transcriptome-based predictions. This analysis allowed the identification of 33,772 genes with an average length of 8,563 bp. The mean coding sequence length was 1,382 bp, with an average of 6.14 exons per gene and an average exon length of 225 bp.
Our comparative genomic analysis considered another scallop species for which a high-quality genome assembly exists (king scallop) and revealed a significant structural divergence between the A. irradians and P. maximus genomes, highlighting a pattern consistent with chromosomal fusion events. Our results revealed the presence of 16 chromosome pairs in A. irradians, consistent with previous karyotype evidence59. These findings support chromosomal rearrangements and fusions. For instance, scaffolds Ai1, Ai2, and Ai3 of A. irradians exhibit syntenic blocks that align with several chromosome-scale scaffolds of P. maximus (Fig. 2c), supporting the hypothesis that these chromosomes are products of an ancestral chromosomal fusion60. This finding is consistent with the observed reduction in chromosome number from the ancestral 1920, aligning with previous studies on chromosomal evolution within the Pectinidae61. The estimated time of divergence of A. irradians and A. purpuratus ~14 million years ago is consistent with fossil data which suggests their separation occurred during the Miocene epoch62. Notably, A. irradians59,63 and A. purpuratus both exhibit a haploid number of 16 chromosomes, deviating from the ancestral state and indicating a lineage-specific reduction. The selective advantage of chromosomal fusions, such as the creation of new gene linkages or the loss of redundant genetic material, is consistent with the concept of local adaptation and the evolution of chromosome fusions64.
Phylogenetic analysis and divergence time estimation
The genome of A. irradians NY and ten other molluscan genomes were used for gene family construction using OrthoFinder v. 2.5.565 with default parameters. The protein sequences of 281 single-copy orthologs from 11 species were independently aligned using MUSCLE66, curated using Gblocks v. 0.91b67 with an option to allow gap positions within the final blocks, and then concatenated using PhyloSuite v. 1.2.268 for species tree construction. The maximum likelihood tree was calculated using IQ-TREE69, based on the recommendations of ModelFinder70, and branching support was estimated using UFBoot71. BEAST 2 v. 2.7.572 was used to estimate species divergence times with the JTT substitution model and gamma categories equal to 4. The calibrated Yule model and strict clock type were set. The chain length for MCMC was set to 10,000,000 and the parameters were recorded every 1,000 generations. The calibration points used in BEAST 2 were obtained from the TimeTree database73: Octopus bimaculoides versus Bivalvia (median time: 520 MYA), Crassostrea virginica versus Magallana gigas (median time: 73 MYA). The gene-family expansion and contraction were determined using CAFE574. The gene family size for each species used in CAFE was calculated by OrthoFinder v. 2.5.565. Comparative genomic analysis of 11 molluscan species, including A. irradians, has revealed major evolutionary events and gene expansions and contractions (Fig. 3). The phylogenetic timeline derived from shared gene sets estimates the divergence of A. irradians and A. purpuratus between 12.6 and 15.9 million years ago (Fig. 3a). Gene clustering analysis using OrthoFinder revealed 7,036 gene families shared among all analyzed molluscan genomes. Among shared gene families, a higher occurrence of shared genes was represented in only one copy within the genus Argopecten, where 84.3–84.4% of the shared gene families were single-copy. It was also found that 1,168 genes in A. irradians and 858 genes in A. purpuratus were clustered into 640 gene families exclusive to Argopecten species and 1792 gene families found in Pectinidae species (Fig. 3b). The A. irradians genome exhibits an expansion of 420 gene families. Enrichment analysis of these families reveals the most substantial increase noted in the phagosome pathway (Fig. 3c).
Gene family dynamics and functional enrichment in molluscan evolution. (a) Phylogenetic analysis and gene family evolution of 11 molluscan species. Tree topology with estimated divergence times (million years ago, MYA, including range) is shown next to each lineage (blue). Number of expanded (green) and contracted (red) gene families shown next each branch. The right panel illustrates the distribution of gene families among 11 molluscan species. It shows the number of shared single-copy genes (red), which are present in all analyzed genomes but exist in only one copy per individual genome; shared multiple-copy genes (yellow), which are present in all analyzed genomes and exist in multiple copies in individual genomes; genes found only in Argopecten species (green); genes found in Pectinidae species (purple); genes found only in the corresponding species (blue); and other species-combination of orthologous genes (grey). (b) Venn Diagram graph of orthologous gene families shared/not shared among 11 molluscan species. (c) Enriched KEGG pathway analysis for expanded gene families in Argopecten irradians. The bubble plot illustrates the top 20 enriched pathways, where the bubble size reflects the number of genes and the color gradient (-log10(P-value)) indicates the significance of the enrichment.
Enriched pathways include amino sugar and nucleotide sugar metabolism, glycosaminoglycan biosynthesis - chondroitin sulfate, and the phosphatidylinositol signaling system. Additionally, we observed significant enrichment in metabolic pathways, such as fatty acid metabolism, arachidonic acid metabolism, and drug metabolism involving cytochrome P450 enzymes.
Data Records
The raw sequencing data and genome assembly of A. irradians have been deposited at the National Center for Biotechnology Information (NCBI) under BioProject PRJNA1050236. The assembled genome has been deposited in the NCBI assembly with the accession number JAYEEO00000000075. The raw PacBio, Illumina Hi-C, and transcriptome data have been deposited in the Sequence Read Archive (SRA) repository with the accession number of SRP47822076. Additionally, the results of annotation have been deposited in the Figshare77 and Dryad78 databases.
Technical Validation
Quality of the final assembly was evaluated using the Benchmarking Universal Single-Copy Orthologs (BUSCO v. 5.3.0)79 analysis with the Metazoa_odb10 lineage. We found 96.2% complete (among which 1.9% are duplicated), and 98.3% complete + fragmented, BUSCO core genes represented in the Metazoa (odb10) BUSCO database (Table 3). Additionally, BUSCO analysis was performed on the annotated proteins, yielding 94.8% complete BUSCOs (of which 2.8% are duplicated), further supporting the quality of the genome annotation.
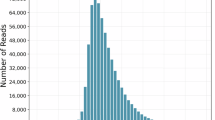
The high quality of the genome assembly is demonstrated by the successful mapping of 96.94% ± 1.61% of transcriptomic reads, as well as second and third generation sequencing data, with mapping rates of 97.72% and 95.87%, respectively (Supplementary Table 1).
Code availability
All analyses followed the guidelines provided in the manuals and tutorials for the software and pipeline used. The specific software versions used are detailed in the Methods section. Default settings or those recommended by the authors were used for the software and analysis pipeline, unless otherwise noted.
References
Adamkewicz, S. L., Harasewych, M. G., Blake, J., Saudek, D. & Bult, C. J. A molecular phylogeny of the bivalve mollusks. Molecular Biology and Evolution 14, 619–629 (1997).
Cummings, K. S. & Graf, D. L. Mollusca. in Ecology and Classification of North American Freshwater Invertebrates 309–384 (Elsevier, 2010).
Strehse, J. S. & Maser, E. Marine bivalves as bioindicators for environmental pollutants with focus on dumped munitions in the sea: A review. Marine Environmental Research 158, 105006 (2020).
Chahouri, A., Yacoubi, B., Moukrim, A. & Banaoui, A. Bivalve molluscs as bioindicators of multiple stressors in the marine environment: Recent advances. Continental Shelf Research 264, 105056 (2023).
Jørgensen, C. Bivalve filter feeding revisited. Mar Ecol Prog Se. 142, 287–302 (1996).
The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. (FAO, Rome, 2022).
Minchin, D. Introductions: some biological and ecological characteristics of scallops. Aquatic Living Resources 16, 521–532 (2003).
Zhan, A. et al. Fine-scale population genetic structure of Zhikong scallop (Chlamys farreri): do local marine currents drive geographical differentiation? Mar Biotechnol 11, 223–235 (2009).
Adamkewicz, L. & Castagna, M. Genetics of shell color and pattern in the bay scallop Argopecten irradians. Journal of Heredity 79, 14–17 (1988).
Estabrooks, S. L. The possible role of telomeres in the short life span of the bay scallop, Argopecten irradians irradians (Lamarck 1819). Journal of Shellfish Research 26, 307–313 (2007).
Guderley, H. E. & Tremblay, I. Swimming in scallops. in Developments in Aquaculture and Fisheries Science vol. 40 535–566 (Elsevier, 2016).
Bert, T. M., Arnold, W. S., McMillen-Jackson, A. L., Wilbur, A. E. & Crawford, C. Natural and anthropogenic forces shape the population genetics and recent evolutionary history of eastern United States bay scallops (Argopecten irradians). Journal of Shellfish Research 30, 583–608 (2011).
Waller, T. R. The evolution of the Argopecten gibbus stock (Mollusca: Bivalvia), with emphasis on the tertiary and quaternary species of Eastern North America. J. Paleontol. 43, 1–125 (1969).
Bologna, P., Wilbur, A. E. & Able, K. Reproduction, population structure, and recruitment limitation in a bay scallop (Argopecten irradians Lamarck) population from New Jersey, USA. Journal of Shellfish Research 20, 89–96 (2001).
Fusui, Z. et al. Introduction, spat-rearing and experimental culture of bay scallop, Argopecten irradians Lamarck. Chin. J. Ocean. Limnol. 9, 123–131 (1991).
Yu, L. et al. Value chain of the data-poor Chinese bay scallop aquaculture. Marine Policy 150, 105556 (2023).
Guo, X. & Luo, Y. Scallops and scallop aquaculture in China. in Developments in Aquaculture and Fisheries Science vol. 40 937–952 (Elsevier, 2016).
Zhang, G. et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490, 49–54 (2012).
Sun, J. et al. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nat Ecol Evol 1, 0121 (2017).
Wang, S. et al. Scallop genome provides insights into evolution of bilaterian karyotype and development. Nat Ecol Evol 1, 120 (2017).
Li, Y. et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat Commun 8, 1721 (2017).
Li, C. et al. Draft genome of the Peruvian scallop Argopecten purpuratus. GigaScience 7, (2018).
Kenny, N. J. et al. The gene-rich genome of the scallop Pecten maximus. GigaScience 9, giaa037 (2020).
Fletcher, C. et al. The genome sequence of the variegated scallop, Mimachlamys varia (Linnaeus, 1758). Wellcome Open Res 8, 307 (2023).
Liu, X. et al. Draft genomes of two Atlantic bay scallop subspecies Argopecten irradians irradians and A. i. concentricus. Sci Data 7, 99 (2020).
Wang, L., Zhang, H., Song, L. & Guo, X. Loss of allele diversity in introduced populations of the hermaphroditic bay scallop Argopecten irradians. Aquaculture 271, 252–259 (2007).
Pales Espinosa, E. et al. An apicomplexan parasite drives the collapse of the bay scallop population in New York. Sci Rep 13, 6655 (2023).
Mathur, V. et al. Phylogenomics identifies a new major subgroup of Apicomplexans, Marosporida class nov., with extreme apicoplast genome reduction. Genome Biology and Evolution 13, evaa244 (2021).
Sambrook, J., Fritsch, E. F., Maniatis, T., Russell, D. W. & Green, M. R. Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989).
Guiglielmoni, N., Houtain, A., Derzelle, A., Van Doninck, K. & Flot, J.-F. Overcoming uncollapsed haplotypes in long-read assemblies of non-model organisms. BMC Bioinformatics 22, 303 (2021).
Vaser, R. & Šikić, M. Time- and memory-efficient genome assembly with Raven. Nat Comput Sci 1, 332–336 (2021).
Roach, M. J., Schmidt, S. A. & Borneman, A. R. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinformatics 19, 460 (2018).
Kundu, R., Casey, J. & Sung, W.-K. HyPo: Super Fast & Accurate Polisher for Long Read Genome Assemblies. https://doi.org/10.1101/2019.12.19.882506 (2019).
Matthey-Doret, C. et al. Computer vision for pattern detection in chromosome contact maps. Nat Commun 11, 5795 (2020).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359 (2012).
Baudry, L. et al. instaGRAAL: chromosome-level quality scaffolding of genomes using a proximity ligation-based scaffolder. Genome Biol 21, 148 (2020).
Laetsch, D. R. & Blaxter, M. L. BlobTools: Interrogation of genome assemblies. F1000Res 6, 1287 (2017).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595 (2010).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Pruitt, K. D., Tatusova, T. & Maglott, D. R. NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 33, D501–504 (2005).
Flynn, J. M. et al. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl. Acad. Sci. USA 117, 9451–9457 (2020).
Benson, G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research 27, 573–580 (1999).
Tarailo-Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinformatics Chapter 4, 4.10.1-4.10.14 (2009).
Stanke, M. et al. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Research 34, W435–W439 (2006).
Majoros, W. H., Pertea, M. & Salzberg, S. L. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics 20, 2878–2879 (2004).
Korf, I. Gene finding in novel genomes. BMC Bioinformatics 5, 59 (2004).
Keilwagen, J. et al. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res 44, e89–e89 (2016).
Kirilenko, B. M. et al. Integrating gene annotation with orthology inference at scale. Science 380, eabn3107 (2023).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37, 907–915 (2019).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33, 290–295 (2015).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol 9, R7 (2008).
Jones, P. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 30, 1236–1240 (2014).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol 428, 726–731 (2016).
Cantalapiedra, C. P., Hernández-Plaza, A., Letunic, I., Bork, P. & Huerta-Cepas, J. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Molecular Biology and Evolution 38, 5825–5829 (2021).
Huerta-Cepas, J. et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Research 47, D309–D314 (2019).
Lagesen, K. et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Research 35, 3100–3108 (2007).
Lowe, T. M. & Eddy, S. R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Research 25, 955–964 (1997).
Nawrocki, E. P., Kolbe, D. L. & Eddy, S. R. Infernal 1.0: inference of RNA alignments. Bioinformatics 25, 1335–1337 (2009).
Huang, X. et al. Cytogenetic characterization of the bay scallop, Argopecten irradians irradians, by multiple staining techniques and fluorescence in situ hybridization. Genes Genet Syst 82, 257–263 (2007).
Wang, Y. & Guo, X. Chromosomal rearrangement in Pectinidae revealed by rRNA loci and implications for bivalve evolution. The Biological Bulletin 207, 247–256 (2004).
Zhang, L., Bao, Z., Wang, S., Huang, X. & Hu, J. Chromosome rearrangements in Pectinidae (Bivalvia: Pteriomorphia) implied based on chromosomal localization of histone H3 gene in four scallops. Genetica 130, 193–198 (2007).
Waller, T. R. The evolution of the Argopecten gibbus stock (Mollusca: Bivalvia), with emphasis on the tertiary and quaternary species of Eastern North America. Memoir (The Paleontological Society) 3, i-v+1–125 (1969).
Gajardo, G., Parraguez, M. & Colihueque, N. Karyotype analysis and chromosome banding of the Chilean-Peruvian scallop Argopecten purpuratus (Lamarck, 1819). J. Shellfish Res 21, 585–590 (2002).
Guerrero, R. F. & Kirkpatrick, M. Local adaptation and the evolution of chromosome fusions. Evolution 68, 2747–2756 (2014).
Emms, D. M. & Kelly, S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol 16, 157 (2015).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004).
Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17, 540–552 (2000).
Zhang, D. et al. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources 20, 348–355 (2020).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32, 268–274 (2015).
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14, 587–589 (2017).
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol Biol Evol 35, 518–522 (2018).
Bouckaert, R. et al. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput Biol 10, e1003537 (2014).
Kumar, S., Stecher, G., Suleski, M. & Hedges, S. B. TimeTree: A resource for timelines, timetrees, and divergence times. Molecular Biology and Evolution 34, 1812–1819 (2017).
Mendes, F. K., Vanderpool, D., Fulton, B. & Hahn, M. W. CAFE 5 models variation in evolutionary rates among gene families. Bioinformatics 36, 5516–5518 (2021).
NCBI Nucleotide. http://identifiers.org/nucleotide:JAYEEO000000000.1 (2024).
NCBI Sequence Read Archive. http://identifiers.org/insdc.sra:SRP478220 (2024).
Grouzdev, D. et al. Chromosome-level genome assembly of the bay scallop Argopecten irradians. Figshare https://doi.org/10.6084/m9.figshare.27015544 (2024).
Grouzdev, D. et al. Chromosome-level genome assembly of the bay scallop Argopecten irradians. Dryad https://doi.org/10.5061/dryad.d51c5b09b (2024).
Seppey, M., Manni, M. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol Biol 1962, 227–245 (2019).
Acknowledgements
This work was supported by NSF Grant number IOS-2026358 to B.A., E.P.E. and S.T. Financial support was also provided by the McConnell Family Foundation (B.A., E.P.E., D.G.) and by the New York State Department of Environmental Conservation (B.A., E.P.E.). N.G. and J-F.F. were funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 764840.
Author information
Authors and Affiliations
Contributions
E.P.E., B.A. and S.T. designed the study and secured the funding. S.T., H.T. and E.P.E. collected and processed biological samples. A.T. and I.B. generated Hi-C libraries. N.G. and J.-F.F. assisted with Hi-C data analysis. D.G., S.F. performed genomic analysis. D.G., E.P.E. and B.A. analyzed data and drafted the paper. All authors contributed to the editing of the manuscript and approved the final version of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Grouzdev, D., Pales Espinosa, E., Tettelbach, S. et al. Chromosome-level genome assembly of the bay scallop Argopecten irradians. Sci Data 11, 1057 (2024). https://doi.org/10.1038/s41597-024-03904-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-024-03904-x