Welcome to the Summer edition of the newsletter.
As Singapore moves to Phase 2 of the "circuit breaker" we have started to (slowly) get back to our routines. Just as we get back to normalcy, so does ISPE Singapore. Our famous conference has a big announcement to accommodate the new ways of working, more events are being added to the schedule, and the technical talks keep getting better!
In this edition of the newsletter, please make sure to check out some of the top articles from our editorial team. Your ISPE membership gives you many privileges, make sure to take advantage of all the offerings that ISPE has to give! In addition, we hear from Vivien Santillan, APAC / Philippines Chair and long time ISPE Australasia board member Benjamin Sauer.
Wishing you and your families best of health during these difficult times, stay safe!
Best regards
Geoffrey Brown, Editor
ISPE Singapore Affiliate
|
 |
 |
Editor's Pick
Don't miss the latest updated guidance documents
Spotlight: Water and Steam Systems Baseline Guide with a message from co-lead Nissan Cohen
Get involved! Women in Pharma
ISPE Communities of Practice
- CoPs act as a knowledge resource by providing a library of articles, presentations, white papers and more grouped according to your technical focus.
- CoPs provide tips that will help you and your company be more successful and productive
- By joining a Community of Practice, you not only gain access to a wealth of knowledge from your colleagues but now you have the opportunity to share your own knowledge and experiences as well!
Here's a handy update from UK affiliate on COVID Resources
|
 |
 |
Interview with... 
Benjamin Sauer
Site Technical and Portfolio Lead, AstraZeneca Australia & Past President, ISPE Australasia Affiliate
ISPE SG: What are some of the lessons that you have learned from your leadership roles in the ISPE Australasia affiliate?
BS: Working with the
ISPE Australasia affiliate has been a great experience over the last 7 years. I have built lasting friendships, developed a robust network, and continued my life-long learning whilst giving back to the Australian Pharmaceutical Industry.
To call out a specific area, the opportunity has helped uplift my change management and stakeholder management skills. Working with a diverse group of volunteers with noble motivation, means that you need to be truly inclusive to ensure you get the best for the local membership and the ISPE volunteer team. Also keeping up to date with relevant industry trends is ever important in ensuring you maintain currency where it matters. Especially in this rapidly changing environment.
ISPE SG: What are some of the opportunities & challenges in your current position?
BS: I am definitely blessed to work for a company like AstraZeneca, whose values align closely with mine. A company that puts the patient first, and challenges me to do my best everyday is truly rewarding. In my current role as Site Technical and Portfolio Lead, my remit includes facilitating strategy execution, portfolio and change management. It is a utility role within the Site Leadership Team that requires a diverse understanding of Pharmaceutical Manufacturing. It is a huge advantage being in a network like ISPE, keeping me up to date with current industry trends, regulatory and guidance, and new technologies. For example, knowing when new ISPE guides are published, like the ISPE data integrity guides, keeps you on the cutting edge. Also, leading the Lean Culture change at North Ryde, the ISPE Cultural Excellence reports provided great insight into what drives success and sustainability of OpEx in an organization.
ISPE SG: As more of our personal and professional lives go online, what are some of the ways you are adapting?
BS: Prior to Covid-19, I never thought that I would personally be a convert to flexible and remote working. However, I readily accepted the change; as much as I do miss the full conversation of face to face interaction, I welcome the change as it has been quite successful for me. One area I have been focusing on during Covid times, is my team(s)' wellbeing and effectiveness. Having more 1 on 1's, ensuring that they have the tools to be effective with the new ways of working, and to ensure my team continues to build trust and capability.
ISPE SG: What are some of the trends you are seeing in operations management within the field of pharmaceutical management?
BS: I can't go past the rapid changes in digitalisation in the industry. Serialisation to prevent counterfeit medicines, enhanced and realtime data visualisation to drive rapid effective decision making, and development of realtime/rapid microtesting (e.g., using PAT) just to name a few. The industry is becoming more agile, and regulators are starting to listen. This is imperative to make full use of what will provide real patient benefit. Safer, more effective drugs to market when and where they are needed. Covid has also helped all of us learn what is possible when previously unimaginable barriers are presented to us. Remote regulator audits, unbelievable drug developments are now being realised, and previously undiscovered collaborations are in play. Digitalisation is also needed to foster this agility, which the industry needs to secure supply chains in this new world of uncertainty. Robotic Process Automation is also a really cool thing. Anything is becoming possible at the right price and with pace.
ISPE SG: Anything else you would like to share with the ISPE SG newsletter readers?
BS: Being in Asia at this time is really exciting. As the world's most populous continent with rapidly developing economies, this will open many doors in our industry as markets grow at amazing rates. We have to secure ourtalent pipelines to ensure our industry capability grows at rates expected in the digital era. We also need further growth to our supply chain agility in the 'new normal'. With the aim to continue to highlight Asia as a pharmaceutical hub of choice for a global market. Networks like ISPE have an important role in this equation.
|
 |
 |
APAC Message 
Vivien E. Santillan
Chair, ISPE APAC Council,
President (2017-2020), ISPE Philippines Affiliate
& Member, ISPE WIP International Steering Committee
The Covid pandemic going on its 4th month has tested our well-being, not just physical but also our mental and even spiritual self. As most countries are transitioning to the "new normal" and slowly opening up its economy, we can't help but still be concerned on what is next but remains to be hopeful.
The ISPE APAC affiliate members postponed or canceled their annual conferences which leads the affiliates to think of innovative and creative means to connect and to continually provide education and knowledge to its members. Webinars and online trainings became the trend where everyone needs to be digitally connected. As we adapt to this platform, we encourage our members and the industry to look out for the different online offerings of each affiliates. On the bright (and economic) side, we get to attend these webinars whether it is hosted in Asia, North America or even in Europe in the comfort of our own home or office. The possibility to learn becomes limitless.
Our resiliency and ingenuity prove that we strive to better our society. Let us diversify our connection and continue not just to network but build relationships. Things are not as easy as before but let us keep going together.
|
 |
 |
VIVA Foundation For Children with Cancer

VIVA ASIA EDUCATION WEBINAR:
Every LCH Child is Different: Unconventional Approaches to a Fascinating Disease
Tuesday, 21 July 2020
20.30 - 21.30 SGT
|
 |
 |
NEW! ISPE Training
Sterile Product Manufacturing Facilities:
Applying the ISPE Baseline® Guide and FDA Guidance Principles to Design and Operation (T12)
Mon 17, Fri 21 Aug 2020 (2-6.30pm) & Mon 24 Aug 2020 (2-5.15pm) | Online
Objectives
This online course uses the third edition of the ISPE Baseline® Guide: Sterile Product Manufacturing Facilities and the FDA's Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing - Current Good Manufacturing Practice to provide an understanding of the key requirements and GMPs for sterile manufacturing facilities. The Guide provides valuable information on design, while the USFDA's Guidance helps professionals understand the regulatory context and expectations for sterile drug manufacturing.
This training will illustrate relevance and applications in the Asian context, with reference to regulatory guidance and practice in the region.
Course Modules
- Introduction to the Baseline Guide
- Concepts and Regulatory Philosophy
- Process and Equipment Considerations
- Architecture and Layout
- Environmental Control Requirements
- HVAC Systems
- Control & Instrumentation
- Commissioning and Qualification
- Barrier-Isolator Technology
- Exercises
- Supplemental Materials
Take Back to Your Job
- Understand the regulatory impact on design, construction, and the regulatory context and expectations for sterile drug manufacturing including the use of RABS and isolator systems
- Identify sources of contamination in aseptic operations
- Explain methods for contamination control
- Describe the major requirements for design, renovation, and operation of a sterile manufacturing facility
- Discuss the fundamentals of aseptic clean room design
- Understand the importance of monitoring critical parameters: temperature, humidity, air velocity, differential pressure, airflow patterns, non-viable particle counts, and microbial counts
- Design a systematic process for aseptic facility layout
- Apply ISO 14644-1:2015, Clean Rooms and Associated Controlled Environments - Part 1: Classification of Air Cleanliness to pharmaceutical manufacturing facilities, and the relationship with the GMP requirements
- Distinguish between the 2004 and 1987 versions of the FDA's Guidance for Industry, Sterile Drug Products Produced by Aseptic Processing - Current Good Manufacturing Practice as applied to the design, operation, maintenance, and modification of facilities
- Engineers, validation scientists, quality assurance specialists, and manufacturing managers
- Professionals who want a fundamental understanding of sterile manufacturing facilities and their design, renovation, and operation
- Engineering firm professionals and other consultants who work with the pharmaceutical industry
Conducted by
Pierre Winnepenninckx, CEO, No deviation, Singapore & Conference Chair, ISPE Singapore Affiliate
Bruce S Davis, Principal, BD Global Consulting
Conducted online, this is a unique opportunity to attend world-class training at an affordable price. In-person attendance usually costs S$ 1,395 in Singapore or US$ 1,495 in USA!
Registration Fee*
S$ 395 / 595 for members / non-members on or before 31 July
or S$ 495 / S$ 695 after
S$ 395 / 595 for members / non-members for groups of 3 or more
S$ 295 for attendees based in Emerging markets / Government / Academia
S$ 80 for students
*does not include Baseline Guide, which is not mandatory but available for purchase
|
 |
|
|
|
|
 |
 |
Technical Tuesday:
Online until CoVid-19 restrictions are eased
Join our regular discussion-based community of practice, open to all and FREE to attend!
Contact us
if you'd like to facilitate a session with a proposed session title and preferred dates.
*Revised speaker & topic!
28 Jul 2020 | 6.30 - 8pm
Case Study: Facilitating Efficient Life-Cycle Management via ICH Q12
Led by Connie S Langer, Associate Director, Pfizer Global Product Development, USA & A
lfonsus Karli, APAC GCMC Advisory Office Director, Pfizer Pte Ltd
& ISPE Singapore Affiliate Vice Chair, Regulatory Affairs
25 Aug 2020 | 6.30 - 8pm
Single-Use Systems in Drug Substance Manufacturing
Led by Miriam O'Sullivan, Senior Quality Manager, Amgen Singapore Manufacturing
29 Sep 2020 | 6.30 - 8pm
Continuous Manufacturing Experience
Led by Thomas P Garcia, Research Fellow in Global CMC, Pfizer & Co-leader, ISPE Continuous Manufacturing Group
27 Oct 2020 | 6.30 - 8pm
Project Management
Led by John Devlin, Founder & CEO, KPC International
17 Nov 2020 | 6.30 - 8pm
Viral Vector Manufacturing
Led by Hugh McKee, Senior Process Consultant, PM Group & ISPE Singapore affiliate Technical Chair

Virtual Sunrise to Sundown
17 Jul 2020 | 10am
Hosted by USA, this will be an interactive and engaging session with games, discussion questions and networking!
Virtual Bookclub
31 Jul 2020 | 10am
Hosted by USA. Please sign up to join us to discuss our first book - How to Win Friends and Influence People In The Digital Age by Dale Carnegie. Time and Virtual meet up link will be sent on July 30th based on Time Zone.
Coffee & Catch-up networking session
8 Aug 2020 | 10 - 11am
Meet your WIP committee and friends during this informal and fun session!
Mentoring & Networking Meet-up
*Subject to change in Covid-19 restrictions
25 Nov 2020 | 2 - 5.30pm
Raffles Marina
WFH Series: Upgrade your Skills Online!
Make the most of this time to improve your productivity with the following sessions.
5 Aug 2020 | 5.30 - 6.30pm
Google Hangouts/Meet/Duo Led by Geoff Brown
Join this social WFH on Google video and chat solutions! The current selection is Google Meet, Google Hangouts and Google Duo. But which app should you be using and what do they each offer? Come join this fun session to learn about the different products and how to use them!
NEW!
ISPE Training
Sterile Product Manufacturing Facilities: Applying the ISPE Baseline® Guide and FDA Guidance Principles to Design and Operation (T12)
Mon 17, Fri 21 Aug 2020 (2 - 6.30pm) & Mon 24 Aug 2020 (2 - 5.15pm) | Online
The training will illustrate relevance and applications in the Asian context, with reference to regulatory guidance and practice in the region. It will employ online tools to ensure a vibrant and interactive experience, with highly engaging speakers.
Conducted online, this is a unique opportunity to attend world-class training at an affordable price. In-person attendance usually costs S$ 1,395 in Singapore or US$ 1,495 in USA!
Registration Fee*:
S$ 395 / 595 for members / non-members on or before 31 July or S$ 495 / S$ 695 after
S$ 395 / 595 for members / non-members for groups of 3 or more
S$ 295 for attendees based in Emerging markets / Government / Academia
S$ 80 for students
*does not include Baseline Guide, which is not mandatory but available for purchase
STERIS Workshop: Points to Consider for Disinfectant Efficacy Studies
8 Sep 2020 | 5 - 6.30pm Online
This presentation will focus on the current regulations, guidelines, method and standards available for disinfectant efficacy studies, the common challenges faced, and how to overcome these challenges.
- Current global regulatory guidance on disinfectant efficacy studies
- Current industry methods for conducting coupon studies
- Points to consider for disinfectant efficacy studies
- Overcoming common challenges
At the end of the presentation, the participants will have a better understanding on the current regulations and guidelines related to disinfectant efficacy studies, the method of performing coupon studies, and also learn about the common challenges faced, and how to overcome these challenges.
Led by
Richard Chai, Technical Service Manager, STERIS Corporation
Hosted by 
Chromatography Workshop: Unlocking downstream process efficiency
*Subject to change in Covid-19 restrictions
25 Sep 2020 | 2 - 5.30pm
In-person, venue to be advised
Each session will be followed by Q&A's for each speaker. It will include a visit to Cytiva's lab in which groups will be split to demo AKTApcc (continuous chromatography), Fibro technology etc.
- Insights into recent development of chromatography resins (30min)
- Unlocking downstream process efficiency with buffer management and continuous chromatography (30min)
- Unlocking downstream process efficiency with automated column packing and single use technologies (30min)
- Smarter process development of chromatography steps (30min)
Hosted by  (formerly GE Healthcare Life Sciences) (formerly GE Healthcare Life Sciences)
ISPE Singapore Bowling Tournament
*Subject to change in Covid-19 restrictions
25 Sep 2020 | 6.30 - 8.30pm
Westwood Bowl @ Civil Service Club, Bukit Batok Clubhouse
Form your own team of 5 or join a team to compete for glory!
Light refreshments and drinks included.
Reduced fees!
Member: S$ 15 |
Non-member: S$ 30 |
Students/YPs/non-bowler: S$ 10

Thirsty Thursday with ISPE!
*Subject to change in Covid-19 restrictions
8 Oct 2020 | 6.30 - 8.30pm
Let's hope that we can catch up in-person before the year ends! Finger food & first 2 rounds on the house!
STERIS Workshop: Steam Sterilization & Autoclave Performance Qualification
20 Oct 2020 | 8 - 9.30pm online
- Sterilization microbiology
- Important elements of autoclave performance qualification
- Concept of using sterilization to control contamination - Biological indicators, chemical indicators and sterilization wrapping
Led by Aaron Mertens, Technical Service Manager, STERIS Corporation
Hosted by 
STERIS Workshop: Cleaning Validation - Sterile Environments Regulatory Trends
10 Nov 2020 | 8 - 9.30pm online
Led by Beth Kroeger, Senior Technical Service Manager, STERIS Corporation
Hosted by 
ISPE Singapore Quiz Night 2020
Dates to be confirmed | 6.30 - 9.30pm
(
Postponed from 23 Apr)
Our long-awaited and most popular annual social event of the year! Food and drinks provided; bring your trivia knowledge along with you!
Registration Fee - S$10 for members / S$40 for non-members or at the door.
Biosafety Workshop
Dates to be confirmed | 2 - 5.30pm
(postponed from 10 Jul 2020)
Genting Hotel Jurong
This introductory workshop will be led by Archlab (Basler & Hofmann). It will cover Biosafety standards and hazards and introduction to a BSL-2 and BSL-3 laboratories. Light refreshments will be provided.
Registration Fee - S$10 for members / S$40 for non-members or at the door.
Register your interest here
ISPE Singapore Conference & Exhibition
* Revised dates & format!
10-12 Dec 2020
*
Online & in-person @ Suntec Convention & Exhibition Centre
With a refreshing new line-up of case studies, extended panel discussions, latest technology and expertise. This year's format will integrate virtual exhibition and interactive live streaming with in-person attendance, so you can join from wherever you are.
View the latest updates and register before the Early Bird deadline to save more and access exclusive preview sessions!
Contact us if you'd like to include your event here. (Postings are vetted according to ISPE guidelines on commercial content and subject to approval by the committee.)
|
 |
|
Clinical Trials and Drug Development in the Time of Covid-19
30 Jul 2020 | 6 - 7.30pm
Please note the following Affiliate Annual Meetings are cancelled:
Thailand 15-16 July 2020
Indonesia 15-16 September 2020
India 6-7 November 2020
India affiliate has hosted 23 recorded free webinars with 26 more to come between now and November!
|
|
|
|
|
 |
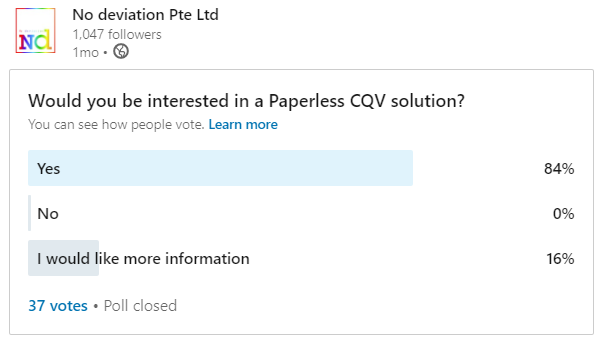
100% of you are interested in Paperless validation.
 Established in 2007, Nd are best known to our MNC and local clients as a long-term partner in the provision of Commissioning
, Qualification and Validation support services - both within Singapore and globally.
As standard bearers for ISPE in the region, Nd has been at the core of many of the discussions around Pharma 4.0.
As a result, in 2018
No deviation
partnered with
Kneat
to bring our clients the best combination of the latest CQV tools coupled with our extensive project execution knowledge.
As part of our journey of Continuous Improvement we're constantly searching new ways to enhance how we support your needs, identifying and trialling unique solutions relevant to your project and quality requirements.
 This is why we have partnered with
Mirrhia a unique Environmental Monitoring System which does not require any coding and yet can integrate equipment and room data - including particle counters & automatic Micro Bio samplers for centralised monitoring and results.
Follow us on
LinkedIn or connect with u
s,
No deviation
today to learn more about Kneat, Mirrhia or to stay updated on our free education sessions & webinars.
|
 |

No deviation webinar: CIP Behind the Scene
13 Jul 2020
Led by Pierre Winnepenninckx, CEO, No Deviation & ISPE Singapore Affiliate Conference Chair and Shan Shan Liu, President, ISPE Singapore affiliate. Over 60 participants gained a holistic view of CIP from Engineering, Science, as well as Compliance perspectives.
View the webinar here
|
Technical Tuesday: QbD (Quality by Design) & PAT (Process Analytical Te
chnology) : Challenges and Opportunities
30 Jun 2020
Thanks to session leader, Dr Chew Wee, A*Star & ISPE Singapore affiliate Subject Matter Expert, PAT
|
STERIS Workshop: Annex 1 draft, Contamination Control Strategy, an Implementation Approach
26 Jun 2020
Thanks to Walid El Azab, Senior Manager, Technical Services, STERIS Life Sciences, Belgium for leading this webinar. Over 100 attendees took part, from Singapore and overseas, including Malaysia, Indonesia, Philippines, Vietnam and Turkey!
|

Women In Pharma Mentoring Evening
27 May 2020
Lively discussion centered on mentoring, expectations and what's in store for mentors and mentees. Thanks to Tanya Sharma for sharing her experience with the team!
View photos here
|
Technical Tuesday: Automation - Most Common Design Issues
26 May 2020
Thanks to session leader, Michael Krispin, Principal Automation Engineer, Integrix Solutions
|
Technical Tuesday: Cell & Gene Therapy - Engineering Perspective
28 Apr 2020
Thanks to session leader, Hugh McKee, Senior Process Consultant, PM Group & ISPE Singapore affiliate Technical Chair
|

WFH Series
Thanks to our session leaders who prepared and ran these interactive and lively workshops to expand our soft skills!
11 Jun 2020
MS-Teams, OneNote Led by Geoff Brown
30 Apr 2020
MS-Word & PPT Led by Geoff Brown
23 Apr 2020
Zoom meetings Led by Hugh McKee
21 Apr 2020
MS-Excel & Airtable Led by Pierre Winnepenninckx
|
Your feedback and suggestions are most welcome as we strive to provide you with membership and industry news updates.
|
|
Contact Us
ISPE Singapore Affiliate
Ph +65 6826 1105
|
|
|
|