|
What’s New? Announcing FIVE (5) new application notes for fecal Calprotectin (fCAL) testing on YOUR Clinical Chemistry analyzer!
While there are over 20 application notes availed in Europe and Canada, please find our FIVE new application notes, fully validated under FDA guidelines for BUHLMANN fCAL® turbo, on the following analyzers:
If your laboratory wants an evaluation, email us today at info@buhlmannlabs.com. Or if you are a clinician, send this email to your laboratorian and ask them to bring fCAL live to your hospital! Announcing new Application notes for fecal Elastase (fPELA)
Applications Notes are now available for:
Soon available:
Learn More:
To request an evaluation for your laboratory, or if you are interested in another application note for your lab’s analyzer, please contact:
Jennifer at jls@buhlmannlabs.com or Collin at cms@buhlmannlabs.com, Ph: (844) 300-9799. 
Technical News Attention all Melatonin customers BÜHLMANN Melatonin assays discontinued, and manufacturing and sales are now transitioned to NovoLytiX GmbH, CEO Dr. Jacky Weber, info@novolytix.ch.
Starting in early 2020, BÜHLMANN evaluated transfer options for its melatonin assay portfolio including the Melatonin RIA assays (RK-DSM2-U and RK-MEL2-U), the Direct Saliva Melatonin ELISA (EK-DSM-U) and the 6-Sulfatoxymelatonin ELISA (EK-M6S-U). As a result, BÜHLMANN Laboratories AG and Swiss based NovolytiX GmbH signed a transfer agreement in July 2020. Both parties agreed, NovolytiX GmbH will take over all melatonin immunoassay kits as of January 1, 2021
Email us at info@buhlmannlabs.com or call (844) 300-979 if you or lab has NOT been contacted about this transition &/or you would like more information. Announcing new Health Canada license for the following BÜHLMANN assays - 1. fecal Elastase assay - BÜHLMANN fPELA® turbo
2. Anti-Infliximab assay - BÜHLMANN Quantum Blue® Anti-Infliximab
For a complete listing of BÜHLMANN regulatory approvals, all of our product landing pages list the Health Canada and FDA regulatory status at https://buhlmannlabs.com or email/phone:
Jennifer at jls@buhlmannlabs.com or Collin at cms@buhlmannlabs.com, Ph: (844) 300-9799. Upcoming Events Resources and Research Announcing BÜHLMANN News update on use of Basophil Activation in oral immunotherapy (Flow CAST® is for research use only in USA): Food allergy is a growing public health burden in children and adults all over the world. In some countries, up to 10% of infants are affected by food allergies (Loh et al., 2018). Relatively few foods like cow milk, egg, wheat, soy, nuts, fish, and shellfish account for the majority of allergies. Nevertheless, every food at any age can cause allergic reactions, from mild symptoms such as itchiness up to serious reactions including anaphylaxis….. If you're interested in Basophil Activation Testing for your lab, email/ phone to Jennifer at jls@buhlmannlabs.com or Collin at cms@buhlmannlabs.com, Ph: (844) 300-9799. Recent Citations Please find a handful of the most recent and stirring references listed below, or visit https://buhlmannlabs.com/category/buhlmann-citations/. [A number of the publications in the citation list describe applications that are outside of product claims thus they should be used for information only.] 1) Duan L, Celik A, Hoang JA, Schmidthaler K, So D, Yin X, Ditlof CM, Ponce M, Upton JEM, Lee JS, Hung L, Breiteneder H, Palladino C, Atkinson AR, Kim VHD, Berenjy A, Asper M, Hummel D, Wong S, Alexanian-Farr M, Magder A, Chinthrajah SR, Mukai K, Tsai M, Nadeau K, Galli SJ, Ramani AK, Szepfalusi Z, Eiwegger T. Basophil activation test shows high accuracy in the diagnosis of peanut and tree nut allergy: The Markers of Nut Allergy Study. Allergy. 2020 Dec 9. doi: 10.1111/all.14695. Epub ahead of print. PMID: 33300157.
“In conclusion, the MONAS study demonstrates that the BAT is effective in diagnosing peanut and tree nut allergy in a real-world setting, and may be useful in reducing the number of resource-intensive OFCs needed. Additional prospective studies to investigate the ability of the BAT to reduce OFCs are required.” Flow CAST® 2) Garnett, E., Pagaduan, J., Rajapakshe, D., Tam, E., Kellermayer, R., & Devaraj, S. (2020). Validation of the newly FDA-approved Buhlmann fCal Turbo assay for measurement of fecal calprotectin in a pediatric population. Practical laboratory medicine, 22, e00178. PMID: 33134465 DOI: 10.1016/j.plabm.2020.e00178
“The newly FDA-cleared fCal Turbo assay, with the Calex cap for sample extraction, represented a much more rapid and facile workflow for our laboratory than referring samples for analysis by ELISA. We determined that the fCal Turbo assay on the Vitros 5600 analyzer offered excellent performance relative to ELISA, was reproducible, and was not excessively prone to analytical interference. We anticipate that it will be a useful tool for identification of IBD in our patient population.” BÜHLMANN fCAL® turbo 3) Isak Östlund , Mårten Werner & Pontus Karling (2020). Self-monitoring with home based fecal calprotectin is associated with increased medical treatment. A randomized controlled trial on patients with inflammatory bowel disease, Scandinavian Journal of Gastroenterology 2021 Jan;56(1):38-45. PMID: 33284639, DOI: 10.1080/00365521.2020.1854342
"Unlike other studies, our study shows that compliance to the IBD-Home model was associated with increased medical treatment and on the short basis with more contacts with health services." IBDoc® 4) D'Amico F, Netter P, Baumann C, Veltin M, Zallot C, Aimone-Gastin I, Danese S, Peyrin-Biroulet L. (2020). Setting up a Virtual Calprotectin Clinic in Inflammatory Bowel Diseases: Literature Review and Nancy Experience. J Clin Med. 2020 Aug 20;9(9):2697.
“The IBDoc® is a simple tool to use and high satisfaction is found among IBDoc® users. IBD patients should be adequately informed and trained on the use of this test. FC home tests are an additional value for e-health approach in IBD patients. In the near future, these tests could allow not only tight monitoring of IBD patients but also their greater involvement in disease management.” IBDoc® BÜHLMANN presented three posters on the high quality of the at this year’s virtual AACC 2020 (American Association of Clinical Chemistry)
5) 20-AACC Poster- Winter et al., Analytical Performance of BÜHLMANN fPELA® turbo, a Fecal Pancreatic Elastase PETIA Assay.
“We believe that BÜHLMANN fPELA® turbo combined with BÜHLMANN CALEX® Cap Extraction Device will further improve the laboratory capability through reduced turn-around time and improved workflow management for random access and high throughput sample processing.” BÜHLMANN fPELA® turbo 6) 20-AACC Poster- Keller et al., First successful comparison of Quantum Blue® rapid TDM assay standardization with WHO international standard for infliximab.
"Current standardization of Quantum Blue® Infliximab rapid test correlates very well with the WHO international standard for infliximab (NIBSC 16/170). This Quantum Blue® Infliximab rapid test represents a unique and modern analytical method, with valid standardization according to WHO for fast time-to-result and simplicity of usage in a more patient near medical environment." BÜHLMANN Quantum Blue® Infliximab 7) 20-AACC Poster- Gernhold et al., A unique rapid test to determine neutralizing antibodies directed against SARS-CoV-2.
BÜHLMANN Quantum Blue® SARS-CoV-2 RBD+ Lateral-Flow assay 8) 20- AACC Poster- E. Troksa, et al., Validation of Fecal Calprotectin (fCal® turbo) by an Automated Routine Chemistry Analyzer Architect C 4000 Platform.
“Conclusion: The Fecal calprotectin fcal® turbo on Architect c4000 is a robust quantitative assay and may meet the Clinical and Laboratory Standard Institute (CLSI) requirements. Fecal calprotectin by PETIA could be proposed in clinical or drug trials as markers for IBD accordingly to the guideline.” BÜHLMANN fCAL® turbo Further Relevant BÜHLMANN citations 9) 20-UEGW Poster- Olson R.N. et al., High Correlation of the Quantum Blue® rapid assay with HPLC tandem mass spectrometry for Infliximab therapeutic drug monitoring. https://youtu.be/N59WxQOCMww
“Lateral flow technology meets mass spectrometry. New data presented at UEG Week 2020 exhibits high correlation of the Quantum Blue® Infliximab rapid test to the gold standard HPLC tandem mass spectrometry method at Mayo clinic (USA)” BÜHLMANN Quantum Blue® Infliximab 9) Kaur S, Ford C, Gama R. BÜHLMANN Calex® Cap for the faecal extraction of calprotectin - Fit for purpose? Ann Clin Biochem. 2020 Jul;57(4):332-333. PMID: 32429682 DOI: 10.1177/0004563220927056
"demonstrated that the Calex CAP extraction devices had a mean bias of 1% relative to the manual weighing method"… "Compared with the manual method, the Calex CAP was easy to use, reduced staff time, used fewer consumables (such as inoculation loops and centrifugation tubes) and improved health and safety by avoiding further direct contact with the specimen after initial sampling.” BÜHLMANN fCAL® turbo For an abbreviated listing of BÜHLMANN assay citations, please https://buhlmannlabs.com/category/buhlmann-citations/ or email or call:
Jennifer at jls@buhlmannlabs.com or Collin at cms@buhlmannlabs.com, Ph: (844) 300-9799. Product Highlights 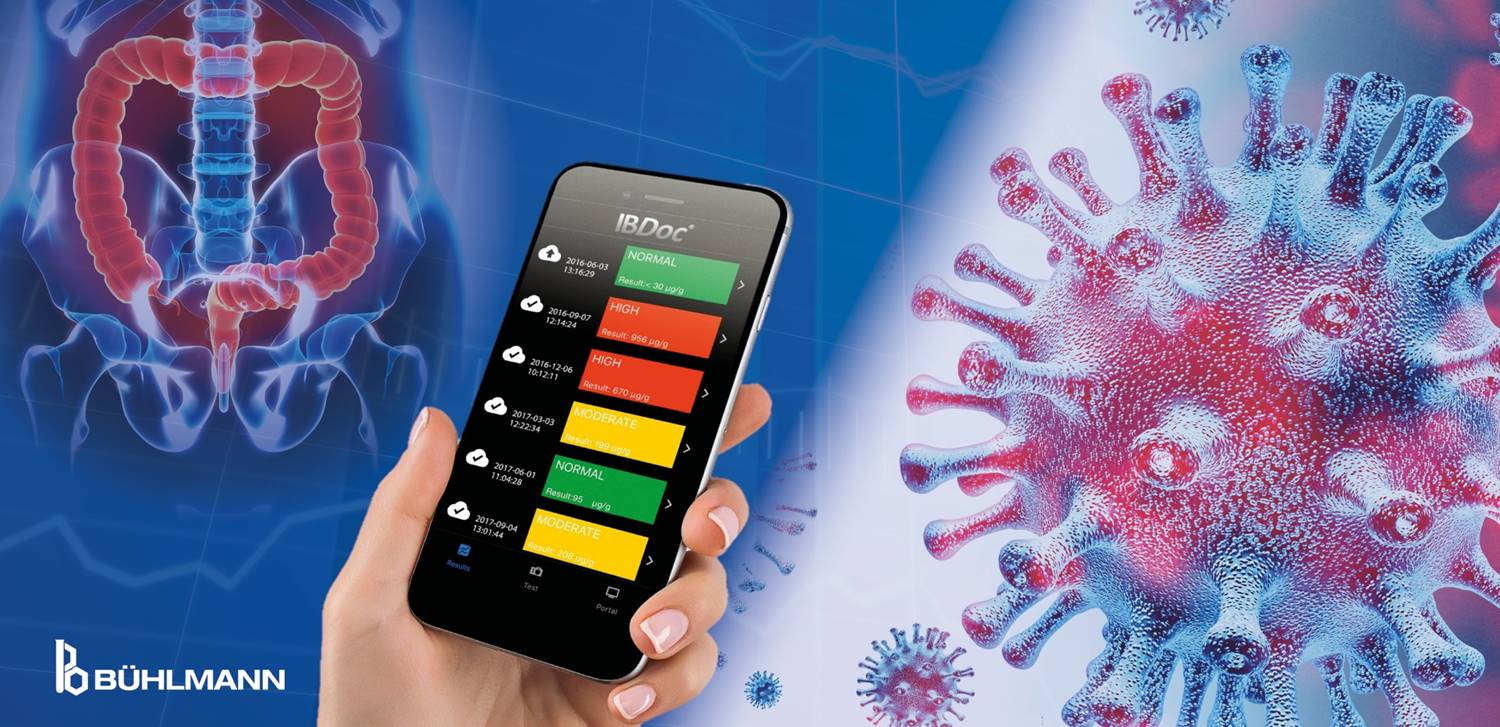
IBDoc®, patient care during COVID-19 [HC Class 3 cleared in Canada, not available for sale in USA]
How does BÜHLMANN help the IBD patient care during the COVID-19 pandemic? In a recent article the task force summarized a list of “Do’s” and “Don’ts” in IBD management.
“Do’s for general clinical practice:
With IBDoc® and the CALEX® Cap Collection Set, BÜHLMANN provides ideal tools for patients to easily measure their calprotectin levels directly at home or send in the stool sample in a safe and convenient way to the laboratory.
IBDoc® can be easily combined with patients self-assessing symptoms through ePRO either app-based, web-based or via communication with the HCP directly. As highlighted in the ECCO taskforce paper, it has been shown as effective as a lab test (Heida et al., 2017) and can provide a rapid assessment of the disease activity while staying at home. Several countries have implemented and successfully tested a combined fCAL home monitoring and ePRO tool e.g. Walsh et al. 2019 or McCombie et al. 2019.
For more insight on how to bring IBDoc® to your hospital or clinic, please email/call Jennifer at jls@buhlmannlabs.com or Collin at cms@buhlmannlabs.com, Ph: (844) 300-9799. BUHLMANN DIAGNOSTICS CORP
BUHLMANN Diagnostic Corp, the North American affiliate of BÜHLMANN Laboratories AG, offers the broadest fecal calprotectin (fCAL) product range in the industry to the United States. These fCAL related test include FDA 510(k) cleared BÜHLMANN fCAL® ELISA, BÜHLMANN fCAL® turbo & CALEX® Cap; FDA exempt BÜHLMANN fPELA® turbo; RUO IBDoc®, Quantum Blue® fCAL rapid tests and Quantum Blue® Infliximab & Adalimumab TDM and Antibody rapid tests. Beyond calprotectin, BDC offers unique assays for Cellular Allergy, Neuroimmunology, Clinical Chemistry and Chronobiology.
Contact Us: 105 Route 101A, Suite 1, Ph: (844) 300-9799 |